| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2008-08-12 14:36:36 UTC |
|---|
| Update Date | 2022-03-07 02:49:33 UTC |
|---|
| HMDB ID | HMDB0006820 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 2-Hexaprenyl-3-methyl-5-hydroxy-6-methoxy-1,4-benzoquinone |
|---|
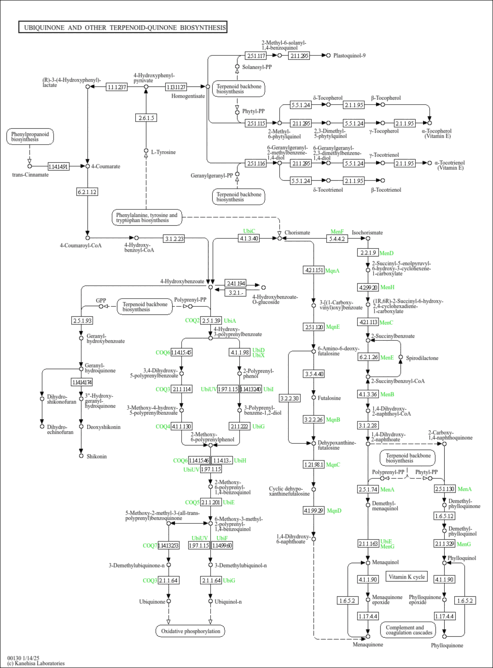
| Description | 2-Hexaprenyl-3-methyl-5-hydroxy-6-methoxy-1,4-benzoquinone is involved in the ubiquinone biosynthesis pathway. 2-Hexaprenyl-3-methyl-5-hydroxy-6-methoxy-1,4-benzoquinone is created from 2-Hexaprenyl-3-methyl-6-methoxy-1,4-benzoquinone by ubiquinone biosynthesis monooxygenase Coq7 [EC:1.14.13.-]. 2-Hexaprenyl-3-methyl-5-hydroxy-6-methoxy-1,4-benzoquinone is then converted to ubiquinone by hexaprenyldihydroxybenzoate methyltransferase [EC:2.1.1.114]. |
|---|
| Structure | COC1=C(O)C(C\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CCC=C(C)C)=C(C)C(=O)C1=O InChI=1S/C38H56O4/c1-27(2)15-10-16-28(3)17-11-18-29(4)19-12-20-30(5)21-13-22-31(6)23-14-24-32(7)25-26-34-33(8)35(39)37(41)38(42-9)36(34)40/h15,17,19,21,23,25,40H,10-14,16,18,20,22,24,26H2,1-9H3/b28-17+,29-19+,30-21+,31-23+,32-25+ |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C38H56O4 |
|---|
| Average Molecular Weight | 576.8488 |
|---|
| Monoisotopic Molecular Weight | 576.41786028 |
|---|
| IUPAC Name | 4-[(2E,6E,10E,14E,18E)-3,7,11,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaen-1-yl]-5-hydroxy-6-methoxy-3-methylcyclohexa-3,5-diene-1,2-dione |
|---|
| Traditional Name | 4-[(2E,6E,10E,14E,18E)-3,7,11,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaen-1-yl]-5-hydroxy-6-methoxy-3-methylcyclohexa-3,5-diene-1,2-dione |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | COC1=C(O)C(C\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CCC=C(C)C)=C(C)C(=O)C1=O |
|---|
| InChI Identifier | InChI=1S/C38H56O4/c1-27(2)15-10-16-28(3)17-11-18-29(4)19-12-20-30(5)21-13-22-31(6)23-14-24-32(7)25-26-34-33(8)35(39)37(41)38(42-9)36(34)40/h15,17,19,21,23,25,40H,10-14,16,18,20,22,24,26H2,1-9H3/b28-17+,29-19+,30-21+,31-23+,32-25+ |
|---|
| InChI Key | IALHZQNPELKEGE-HGJBZHBGSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as sesterterpenoids. These are terpenes composed of five consecutive isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesterterpenoids |
|---|
| Direct Parent | Sesterterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sesterterpenoid
- Quinone
- O-benzoquinone
- Vinylogous acid
- Cyclic ketone
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. | 8.61 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 34.4365 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 0.85 minutes | 32390414 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 5347.8 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 872.7 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 409.1 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 414.1 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 177.2 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 1469.2 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 1410.8 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 102.9 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 3037.5 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 1125.7 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 2061.7 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 1028.8 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 710.4 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 239.4 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 851.0 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 10.0 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - 2-Hexaprenyl-3-methyl-5-hydroxy-6-methoxy-1,4-benzoquinone GC-MS (Non-derivatized) - 70eV, Positive | splash10-06y9-2197580000-76798e00092a2c131caf | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 2-Hexaprenyl-3-methyl-5-hydroxy-6-methoxy-1,4-benzoquinone GC-MS (1 TMS) - 70eV, Positive | splash10-003r-3147469000-8e87818f8a300107677e | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 2-Hexaprenyl-3-methyl-5-hydroxy-6-methoxy-1,4-benzoquinone GC-MS ("2-Hexaprenyl-3-methyl-5-hydroxy-6-methoxy-1,4-benzoquinone,1TMS,#1" TMS) - 70eV, Positive | Not Available | 2021-10-14 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 2-Hexaprenyl-3-methyl-5-hydroxy-6-methoxy-1,4-benzoquinone GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-10-15 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2-Hexaprenyl-3-methyl-5-hydroxy-6-methoxy-1,4-benzoquinone 10V, Positive-QTOF | splash10-004i-0213190000-920b7c37f14e9166fd52 | 2016-09-14 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2-Hexaprenyl-3-methyl-5-hydroxy-6-methoxy-1,4-benzoquinone 20V, Positive-QTOF | splash10-055e-0549230000-d8a6fac96d7d16b96682 | 2016-09-14 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2-Hexaprenyl-3-methyl-5-hydroxy-6-methoxy-1,4-benzoquinone 40V, Positive-QTOF | splash10-00kb-2124910000-a366c27333a192119553 | 2016-09-14 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2-Hexaprenyl-3-methyl-5-hydroxy-6-methoxy-1,4-benzoquinone 10V, Negative-QTOF | splash10-004i-0000190000-3f54bfed75c669f7dc33 | 2016-09-14 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2-Hexaprenyl-3-methyl-5-hydroxy-6-methoxy-1,4-benzoquinone 20V, Negative-QTOF | splash10-00vi-2000190000-f1b2a4654166322d6bb1 | 2016-09-14 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2-Hexaprenyl-3-methyl-5-hydroxy-6-methoxy-1,4-benzoquinone 40V, Negative-QTOF | splash10-05fr-9200760000-68e907ef0147e68a9d30 | 2016-09-14 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2-Hexaprenyl-3-methyl-5-hydroxy-6-methoxy-1,4-benzoquinone 10V, Positive-QTOF | splash10-003r-3319380000-9dc69ff763b6321e9657 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2-Hexaprenyl-3-methyl-5-hydroxy-6-methoxy-1,4-benzoquinone 20V, Positive-QTOF | splash10-001i-4429300000-12764c6c973b0da55978 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2-Hexaprenyl-3-methyl-5-hydroxy-6-methoxy-1,4-benzoquinone 40V, Positive-QTOF | splash10-01po-9416000000-0eefff51a1672a90802a | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2-Hexaprenyl-3-methyl-5-hydroxy-6-methoxy-1,4-benzoquinone 10V, Negative-QTOF | splash10-004i-0000090000-774ca73f3ba2d11a3818 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2-Hexaprenyl-3-methyl-5-hydroxy-6-methoxy-1,4-benzoquinone 20V, Negative-QTOF | splash10-004j-0711090000-843349735a4269148d36 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2-Hexaprenyl-3-methyl-5-hydroxy-6-methoxy-1,4-benzoquinone 40V, Negative-QTOF | splash10-014l-0962220000-a82e4a622f5f27c214ac | 2021-09-22 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | 2022-08-22 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | 2022-08-22 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | 2022-08-22 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | 2022-08-22 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | 2022-08-22 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | 2022-08-22 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | 2022-08-22 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | 2022-08-22 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | 2022-08-22 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | 2022-08-22 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | 2022-08-22 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | 2022-08-22 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | 2022-08-22 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | 2022-08-22 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | 2022-08-22 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | 2022-08-22 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | 2022-08-22 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | 2022-08-22 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | 2022-08-22 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | 2022-08-22 | Wishart Lab | View Spectrum |
|
|---|